Abstract
Background and Scientific Rationale:
Multiple myeloma (MM) is often preceded by the premalignant conditions monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Obesity, low adiponectin levels, and diets high in inflammatory, insulinemic foods or lacking plant-based foods are known risk factors for the development of MGUS/SMM, as well as for progression to MM. Therefore, there is an opportunity to study a dietary intervention in cancer progression among patients with MGUS/SMM, for which the standard of care is observation even though some patients will eventually progress to MM. This is a pilot nutrition-based intervention study of a whole food, plant-based diet (WFPBD) in overweight and obese MGUS and SMM patients to enable weight loss, assess associated changes in disease biomarkers, epigenetics, and the gut microbiome. We expect that the findings will enable larger lifestyle-based studies of prevention and survivorship in plasma cell disorders.
Study Design and Methods:
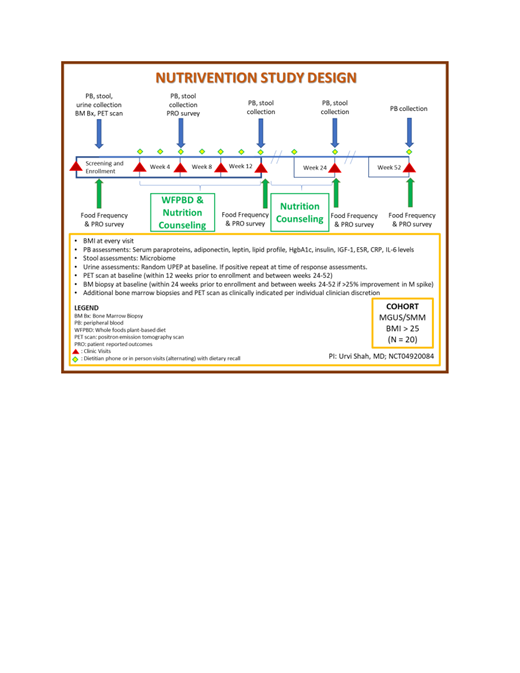
This is a single-arm, single-center pilot study of a WFPBD for 12 weeks and nutrition counseling for 24 weeks which will enroll 20 patients (Figure).
Clinical trial registry number: NCT04920084, actively recruiting.
Study Population and Inclusion Criteria
i) SMM or MGUS
ii) Body mass index ≥25
iii) Monoclonal protein spike ≥0.2 g/dL or abnormal free light chain ratio with increased level of the appropriate involved light chain
iv) ECOG performance status 0-3
v) Willingness to comply with study-related procedures
Statistical Methods:
The average weight loss from baseline at 12 weeks will be reported as sample mean along with 95% confidence interval. Adherence will be estimated by sample proportion, with confidence intervals based on exact binomial distribution. Patients who have completed evaluation at 12 weeks will be evaluable for the weight loss outcome. All patients who have received at least one WFPBD intervention will be evaluable for adherence assessment. We will consider this intervention promising if 1) we detect weight loss at 12 weeks and 2) estimated adherence to the intervention is ≥70%.
Study Treatment:
For 12 weeks, patients will receive two premade meals per day, for lunch and dinner for 6 days per week, prepared and shipped by Plantable. The meals will have a low glycemic index and contain vegetables, whole grains, and plant-based fats that have undergone minimal processing. Detailed recommendations for snacks and breakfasts meeting the standard of a WFPBD will also be given to supplement their daily calorie needs with access to an online portal or phone application from Plantable which contains education materials and access to a coach daily for 24 weeks. Patients will receive dietary education and counseling from a research dietitian every 2 weeks for the 12-week intervention period.
Endpoints:
Primary:
- To determine the feasibility of a WFPBD, as measured by weight loss and adherence at 12 weeks.
Secondary:
- To determine the feasibility of a WFPBD, as measured by safety, and quality of life.
- To assess weight loss at 24, and 52 weeks.
- To assess alterations in metabolic, and myeloma markers.
Exploratory:
- To assess alterations in T cell and plasma cell epigenetic markers
- To assess alterations in the fecal microbiome
- To assess changes in body composition as determined by PET imaging and correlate with weight change as well as disease markers.
Shah: Janssen: Research Funding; Celgene/BMS: Research Funding. Adintori: Vidafuel Inc.: Current holder of stock options in a privately-held company. Mailankody: Jansen Oncology: Research Funding; Physician Education Resource: Honoraria; Bristol Myers Squibb/Juno: Research Funding; Plexus Communications: Honoraria; Fate Therapeutics: Research Funding; Takeda Oncology: Research Funding; Allogene Therapeutics: Research Funding; Legend Biotech: Consultancy; Evicore: Consultancy. Korde: Medimmune: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Hultcrantz: Intellisphere LLC: Consultancy; Amgen: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Research Funding; Curio Science LLC: Consultancy. Hassoun: Celgene, Takeda, Janssen: Research Funding. D'Souza: Sanofi, Takeda, Teneobio, CAELUM, Prothena: Research Funding; Imbrium, Pfizer, BMS: Membership on an entity's Board of Directors or advisory committees; Janssen, Prothena: Consultancy. Giralt: AMGEN: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; Actinnum: Membership on an entity's Board of Directors or advisory committees; JENSENN: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees. Iyengar: Novartis: Consultancy; Seattle Genetics: Consultancy; Novartis: Research Funding. Landgren: Janssen: Other: IDMC; Janssen: Honoraria; Amgen: Honoraria; Celgene: Research Funding; Janssen: Research Funding; Amgen: Research Funding; Takeda: Other: IDMC; GSK: Honoraria. van den Brink: Pluto Therapeutics: Current holder of stock options in a privately-held company, Other: has consulted, received honorarium from or participated in advisory boards ; Novartis (Spouse): Other: has consulted, received honorarium from or participated in advisory boards; Forty-Seven, Inc.: Honoraria; MagentaTherapeutics: Honoraria; Da Volterra: Other: has consulted, received honorarium from or participated in advisory boards; Wolters Kluwer: Patents & Royalties; Ceramedix: Other: has consulted, received honorarium from or participated in advisory boards ; Notch Therapeutics: Honoraria; DKMS (nonprofit): Other; Pharmacyclics: Other; Kite Pharmaceuticals: Other; GlaskoSmithKline: Other: has consulted, received honorarium from or participated in advisory boards; Lygenesis: Other: has consulted, received honorarium from or participated in advisory boards ; Nektar Therapeutics: Honoraria; Rheos: Honoraria; Seres: Other: Honorarium, Intellectual Property Rights, Research Fundingand Stock Options; Amgen: Honoraria; Therakos: Honoraria; WindMILTherapeutics: Honoraria; Juno Therapeutics: Other; Merck & Co, Inc: Honoraria; Frazier Healthcare Partners: Honoraria; Priothera: Research Funding; Synthekine (Spouse): Other: has consulted, received honorarium from or participated in advisory boards; Jazz Pharmaceuticals: Honoraria. Lesokhin: pfizer: Consultancy, Research Funding; Genetech: Research Funding; Trillium Therapeutics: Consultancy; Behringer Ingelheim: Honoraria; Serametrix, Inc: Patents & Royalties; bristol myers squibb: Research Funding; Janssen: Honoraria, Research Funding; Iteos: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal